Your new post is loading...
In one of the largest microbiota studies conducted in humans, researchers atWestern University, Lawson Health Research Institute and Tianyi Health Science Institute in Zhenjiang, Jiangsu, China have shown a potential link between healthy aging and a healthy gut. With the establishment of the China-Canada Institute, the researchers studied the gut bacteria in a cohort of more than 1,000 Chinese individuals in a variety of age-ranges from 3 to over 100 years-old who were self-selected to be extremely healthy with no known health issues and no family history of disease. The results showed a direct correlation between health and the microbes in the intestine. “The aim is to bring novel microbiome diagnostic systems to populations, then use food and probiotics to try and improve biomarkers of health,” said Gregor Reid, PhD, professor at Western’s Schulich School of Medicine & Dentistry and Scientist at Lawson Health Research Institute. “It begs the question – if you can stay active and eat well, will you age better, or is healthy aging predicated by the bacteria in your gut?” The study, published this month in the journal mSphere, showed that the overall microbiota composition of the healthy elderly group was similar to that of people decades younger, and that the gut microbiota differed little between individuals from the ages of 30 to over 100. “The main conclusion is that if you are ridiculously healthy and 90 years old, your gut microbiota is not that different from a healthy 30 year old in the same population,” said Greg Gloor, PhD, the principal investigator on the study and also a professor at Western’s Schulich School of Medicine & Dentistry and Scientist at Lawson Health Research Institute. Whether this is cause or effect is unknown, but the study authors point out that it is the diversity of the gut microbiota that remained the same through their study group. “This demonstrates that maintaining diversity of your gut as you age is a biomarker of healthy aging, just like low-cholesterol is a biomarker of a healthy circulatory system,” Gloor said. The researchers suggest that resetting an elderly microbiota to that of a 30-year-old might help promote health.
Craig Venter’s new company wants to improve human longevity by creating the world’s largest, most comprehensive database of genetic and physiological information.
Human Longevity, based in San Diego, says it will sequence some 40,000 human genomes per year to start, using Illumina’s new high-throughput sequencing machines (Illumina Has the First $1,000 Genome).
Eventually, it plans to work its way up to 100,000 genomes per year. The company will also sequence the genomes of the body’s multitudes of microbial inhabitants, called the microbiome, and analyze the thousands of metabolites that can be found in blood and other patient samples.
By combining these disparate types of data, the new company hopes to make inroads into the enigmatic process of aging and the many diseases, including cancer and heart disease, that are strongly associated with it. “Aging is exerting a force on humans that is exposing us to diseases, and the diseases are idiosyncratic, partly based on genetics, partly on environment,” says Leonard Guarente, who researches aging at MIT and is not involved in the company. “The hope for many of us who study aging is that by having interventions that hit key pathways in aging, we can affect disease.”
To that end, Human Longevity will collaborate with Metabolon, a company based in Durham, North Carolina, to profile the metabolites circulating in the bloodstreams of study participants. Metabolon was an early pioneer in the field of metabolomics, which catalogues the amino acids, fats, and other small molecules in a blood or other sample to develop more accurate diagnostic tests for diseases (Metabolomics).
Metabolon uses mass spectrometry to identify small molecules in a sample. In a human blood sample, there are around 1,200 different types; Metabolon’s process can also determine the amount of each one present. While genome sequencing can provide information about inherited risk of disease and some hints of the likelihood that a person will have a long life, metabolic data provides information on how environment, diet, and other features of an individual’s life affect health.
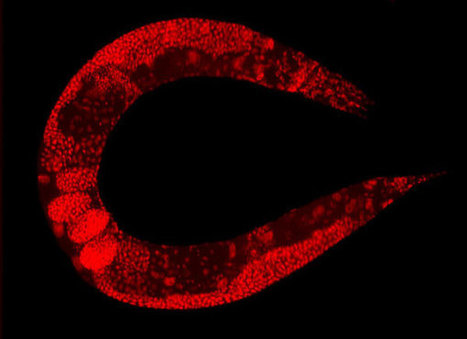
C. elegans worms whose grandparents had the ability to fight viruses using a fleet of tiny RNA molecules retain these molecules even when they don’t have the genes for them. They can pass these molecules down for more than a hundred generations. A research team engineered worms that didn’t have the genes to make the RNAs—which work by inhibiting the virus replication machinery—and then bred them with worms that did for several generations. They ended up with some worms whose ancestors had had the virus-fighting molecules, but did not themselves possess the necessary genes. The team then watched these worms under the microscope and saw that they still attacked viruses in exactly the same way as their grandparents. Numerous control experiments confirmed that the effect was real, and only happened in worms who had ancestors with the genes. The researchers collected all the various RNA molecules in these worms and saw that indeed, they possessed the virus-fighting variety.After about three generations, the effect seemed to wear off; most worms without the genes stopped being able to attack viruses. But for some worms, it never stopped. The team bred those worms for more than one hundred generations, nearly a year, and the creatures never flagged in their ability to defend themselves. How is this possible? The team keeps mum on any ideas of how this inheritance works. But they do uncover some tantalizing details that give us room for speculation. One possibility is that the RNA molecules made by the original worms in response to a virus attack were floating around in the cytoplasm of the eggs and sperm that became their offspring. If that’s the case, then the offspring are basically using their parents’ leftovers, with each generation having a bit less of the original stuff. The researchers mention this possibility of the original RNA being “diluted” with each generation, but don’t, as far as we can tell, try to test that.But what about the worms that hang on to the RNA indefinitely? The researchers found that for that to happen, a particular enzyme that builds RNAs has to be present. Maybe, then, these worms manage to jerry-rig a way to make copies of the virus-fighting RNA with that enzyme (which isn’t part of the usual machinery), even though they lack the gear required to make it in the normal fashion. The gene for that enzyme would then be passed on as normal.
Via Sakis Koukouvis
Last month, a 114-year-old former schoolteacher from Georgia named Besse Cooper became the world's oldest living person. Her predecessor, Brazil's Maria Gomes Valentim, was 114 when she died. So was the oldest living person before her, and the one before her. In fact, eight of the last nine "world's oldest" titleholders were 114 when they achieved the distinction. Here's the morbid part: All but two were still 114 when they passed it on. Those two? They died at 115. Is 115 years the magical limit?
|
The gut microbes of young killifish can extend the lifespans of older fish – hinting at the microbiome’s role in aging. It may not be the most appetizing way to extend life, but researchers have shown for the first time that older fish live longer after they consumed microbes from the poo of younger fish. The findings were posted to the bioRxiv.org preprint server on 27 March1 by Dario Valenzano, a geneticist at the Max Planck Institute for Biology of Ageing in Köln, Germany, and his colleagues. So-called ‘young blood’ experiments that join the circulatory systems of two rats — one young and the other old — have found that factors coursing through the veins of young rodents can improve the health and longevity of older animals. But the new first-of-its-kind study examined the effects of 'transplanting' gut microbiomes on longevity. “The paper is quite stunning. It’s very well done,” says Heinrich Jasper, a developmental biologist and geneticist at the Buck Institute for Research on Aging in Novato, California, who anticipates that scientists will test whether such microbiome transplants can extend lifespan in other animals. Life is fleeting for killifish, one of the shortest-lived vertebrates on Earth: the fish hits sexual maturity at three weeks old and dies within a few months. The turquoise killifish (Nothobranchius furzeri) that Valenzano and his colleagues studied in the lab inhabits ephemeral ponds that form during rainy seasons in Mozambique and Zimbabwe. Previous studies have hinted at a link between the microbiome and aging in a range of animals. As they age, humans2 and mice3 tend to lose some of the diversity in their microbiomes, developing a more uniform community of gut microbes, with once-rare and pathogenic species rising to dominance in older individuals4. The same pattern holds true in killifish, whose gut microbiomes at a young age are nearly as diverse as those of mice and humans, says Valenzano. “You can really tell whether a fish is young or old based on its gut microbiota.” To test whether the changes in the microbiome had a role in ageing, Valenzano’s team ‘transplanted’ the gut microbes from 6-week-old killifish into middle-aged 9.5-week-old fish. They first treated the middle-aged fish with antibiotics to clear out their gut flora, then placed them in a sterile aquarium containing the gut contents of young fish for 12 hours. Killifish don’t usually eat faeces, Valenzano notes, but they would probe and bite at the gut contents to see whether it was food, ingesting microbes in the process. The transplanted microbes successfully recolonized the guts of the fish that received them, the team found. At 16 weeks of age, the gut microbiomes of middle-aged fish that received 'young microbes' still resembled those of 6-week-old fish. The young microbiome ‘transplant’ also had dramatic effects on the longevity of fish that got them: their median lifespans were 41% longer than fish exposed to microbes from middle-aged animals, and 37% longer than fish that received no treatment (antibiotics alone also lengthened lifespan, but to a lesser extent).
Using 17 genomes, researchers were unable to find rare protein-altering variants significantly associated with extreme longevity, according to a study published November 12, 2014 in the open-access journal PLOS ONE by Hinco Gierman from Stanford University and colleagues.
Supercentenarians are the world’s oldest people, living beyond 110 years of age. Seventy-four are alive worldwide; 22 live in the U.S. The authors of this study performed whole-genome sequencing on 17 supercentenarians to explore the genetic basis underlying extreme human longevity.
From this small sample size, the researchers were unable to find rare protein-altering variants significantly associated with extreme longevity compared to control genomes. However, they did find that one supercentenarian carries a variant associated with a heart condition, which had little or no effect on his/her health, as this person lived over 110 years.
Although the authors didn’t find significant association with extreme longevity, the authors have publicly published the genomes, making them available as a resource for future studies on the genetic basis of extreme longevity.
Reference:
A cancer normally lives and dies with a person, however this is not the case with a sexually transmitted cancer in dogs. In a study published in Science, researchers have described the genome and evolution of this cancer that has continued living within the dog population for the past 11,000 years.
Scientists have sequenced the genome of the world's oldest continuously surviving cancer, a transmissible genital cancer that affects dogs. This cancer, which causes grotesque genital tumors in dogs around the world, first arose in a single dog that lived about 11,000 years ago. The cancer survived after the death of this dog by the transfer of its cancer cells to other dogs during mating.
The genome of this 11,000-year-old cancer carries about two million mutations -- many more mutations than are found in most human cancers, the majority of which have between 1,000 and 5,000 mutations. The team used one type of mutation, known to accumulate steadily over time as a "molecular clock," to estimate that the cancer first arose 11,000 years ago.
"The genome of this remarkable long-lived cancer has demonstrated that, given the right conditions, cancers can continue to survive for more than 10,000 years despite the accumulation of millions of mutations," says Dr Elizabeth Murchison, first author from the Wellcome Trust Sanger Institute and the University of Cambridge.
The genome of the transmissible dog cancer still harbors the genetic variants of the individual dog that first gave rise to the cancer 11,000 years ago. Analysis of these genetic variants revealed that this dog may have resembled an Alaskan Malamute or Husky. It probably had a short, straight coat that was colored either grey/brown or black. Its genetic sequence could not determine if this dog was a male or a female, but did indicate that it was a relatively inbred individual.
"We do not know why this particular individual gave rise to a transmissible cancer," says Dr Murchison, "But it is fascinating to look back in time and reconstruct the identity of this ancient dog whose genome is still alive today in the cells of the cancer that it spawned." Transmissible dog cancer is a common disease found in dogs around the world today. The genome sequence has helped scientists to further understand how this disease has spread.
"The patterns of genetic variants in tumors from different continents suggested that the cancer existed in one isolated population of dogs for most of its history," says Dr Murchison. "It spread around the world within the last 500 years, possibly carried by dogs accompanying seafarers on their global explorations during the dawn of the age of exploration."
Transmissible cancers are extremely rare in nature. Cancers, in humans and animals, arise when a single cell in the body acquires mutations that cause it to produce more copies of itself. Cancer cells often spread to different parts of the body in a process known as metastasis. However, it is very rare for cancer cells to leave the bodies of their original hosts and to spread to other individuals. Apart from the dog transmissible cancer, the only other known naturally occurring transmissible cancer is an aggressive transmissible facial cancer in Tasmanian devils that is spread by biting.
Brooke Greenberg (born January 8, 1993), is a now 20 year old girl from Reisterstown, Maryland, who has remained physically and cognitively similar to a toddler. She is about 30 inches (76 cm) tall, weighs about 16 pounds (7.3 kg), and has an estimated mental age of 9 months to 1 year. Brooke’s doctors have termed her condition Syndrome X. Brooke was born on January 8, 1993 at Sinai Hospital in Baltimore, Maryland, one month prior to her due date, weighing just four pounds (1.8 kg). She was born with anterior hip dislocation, a condition which caused her legs to be swiveled upwards, awkwardly, toward her shoulders; this was corrected surgically. Otherwise, Brooke appeared to be a normal infant.
In her first six years, Brooke Greenberg went through a series of unexplained medical emergencies from which she recovered. She had seven perforated stomach ulcers. She also suffered a seizure. This was followed by what was later diagnosed as a stroke; weeks later, no damage was detected. At age five, Brooke had a mass in her brain that caused her to sleep for 14 days. The doctors diagnosed the mass as a brain tumor. However, Brooke later awoke, and physicians found no tumor present. Brooke’s pediatrician, Dr. Lawrence Pakula, states that the source of her sudden illness remains a mystery.
Over the past several years, the Greenbergs visited many specialists, looking for an explanation for their daughter’s strange condition, and found she has a mutation in Chromosome 1. In 2001, when Dateline documented Brooke, she was still the size of a six-month-old infant, weighing just 13 lb (5.9 kg) at 27 inches (69 cm) tall. The family still had no explanation. Brooke Greenberg’s mother Melanie said: “They [the specialists] just said she’ll catch up. Then we went to the nutritionist, the endocrinologist. We tried the growth hormone…”. The growth hormone treatment had no effect. Howard, Brooke’s father, said: “I mean she did not put on an ounce or she did not grow an inch … That’s when I knew there was a problem.” After the growth hormone administration failed, the doctors, unable to diagnose a known condition, named her condition Syndrome X.
The Greenbergs made many visits to nearby Johns Hopkins Children’s Center, and even took Brooke to New York’s Mount Sinai Hospital, searching for information about their daughter’s condition. When geneticists sequenced Greenberg’s DNA, they found that the genes associated with the premature aging diseases were normal, unlike the mutated versions in patients with Werner syndrome and progeria.
In 2006, Dr. Richard Walker of the University of South Florida College of Medicine, said that Brooke’s body is not developing as a coordinated unit, but as independent parts that are out of sync. She has never been diagnosed with any known genetic disorder or chromosomal abnormality that would help explain why.
In 2009, Walker said: “There’ve been very minimal changes in Brooke’s brain … Various parts of her body, rather than all being at the same stage, seem to be disconnected.” Walker noted that Greenberg’s brain, for example, is not much more mature than that of a newborn infant. He estimates her mental age at around 9 months to a year old. Brooke can make gestures and recognize sounds, but cannot speak. Her bones are like those of a ten-year-old, and she still has her baby teeth, which have an estimated developmental age of about 8 years. Said Walker, “We think that Brooke’s condition presents us with a unique opportunity to understand the process of aging.”
Her telomeres seem to be shortening at the normal rate.
Yesterday, Brooke Greenberg and her family appeared on the Katie Couric Show as part of a show focused on medical mysteries. Greenberg, who is 20 years old, appears to be the age of a toddler. While the family does not know the root of Greenberg's apparent inability to age, Mount Sinai's Eric Schadt, who also appeared on the show with host Katie Couric, has been studying her genome. As Schadt notes in the clip below, in addition to figuring out the source of her disorder, Greenberg's genome could also help researchers learn more about the aging process.
Video Link: http://bcove.me/uesqzs6d
The entire DNA sequence of a woman who lived to 115 has been pieced together by scientists. The woman, who was the oldest in the world at the time of her death, had the mind of someone decades younger and no signs of dementia. She appeared to have some rare genetic changes in her DNA, some of them might have protected her against dementia and other diseases of later life. The woman was born prematurely and was not expected to survive. But she lived a long and healthy life, and entered a care home at the age of 105. She eventually died from a stomach tumor, having been treated for breast cancer at the age of 100.
|



 Your new post is loading...
Your new post is loading...