 Your new post is loading...
 Your new post is loading...
CD8+ cytotoxic T cells (CTLs) orchestrate antitumour immunity and exhibit inherent heterogeneity1,2, with precursor exhausted T (Tpex) cells but not terminally exhausted T (Tex) cells capable of responding to existing immunotherapies3–7. The gene regulatory network that underlies CTL differentiation and whether Tex cell responses can be functionally reinvigorated are incompletely understood. Here we systematically mapped causal gene regulatory networks using single-cell CRISPR screens in vivo and discovered checkpoints for CTL differentiation. First, the exit from quiescence of Tpex cells initiated successive differentiation into intermediate Tex cells. This process is differentially regulated by IKAROS and ETS1, the deficiencies of which dampened and increased mTORC1-associated metabolic activities, respectively. IKAROS-deficient cells accumulated as a metabolically quiescent Tpex cell population with limited differentiation potential following immune checkpoint blockade (ICB). Conversely, targeting ETS1 improved antitumour immunity and ICB efficacy by boosting differentiation of Tpex to intermediate Tex cells and metabolic rewiring. Mechanistically, TCF-1 and BATF are the targets for IKAROS and ETS1, respectively. Second, the RBPJ–IRF1 axis promoted differentiation of intermediate Tex to terminal Tex cells. Accordingly, targeting RBPJ enhanced functional and epigenetic reprogramming of Tex cells towards the proliferative state and improved therapeutic effects and ICB efficacy. Collectively, our study reveals that promoting the exit from quiescence of Tpex cells and enriching the proliferative Tex cell state act as key modalities for antitumour effects and provides a systemic framework to integrate cell fate regulomes and reprogrammable functional determinants for cancer immunity. Analyses of causal gene regulatory networks have identified key checkpoints mediating the progressive differentiation of CD8+ cytotoxic T cells, findings that have implications for anticancer immunotherapies such as adoptive cell therapy and immune checkpoint blockade.
Looking to replicate the impressive findings with CAR T-cell therapies observed in patients with hematologic malignancies, investigators have initiated the BASECAMP-1 trial with the hope of identifying patients with advanced solid tumors who will be suitable candidates for treatment in the EVEREST trial.
Researchers of the Hospital Universitario 12 de Octubre in Madrid and the Josep Carreras Leukaemia Research Institute have developed a cell therapy for a type of leukemia which currently has very few treatment options.
The FDA granted orphan drug designation to WU-CART-007 for patients with relapsed or refractory T-cell acute lymphoblastic leukemia.WU-CART-007 (Wugen) is an allogeneic, gene-edited chimeric antigen receptor T-cell therapy that targets the protein CD7 on the surface of cancer cells.
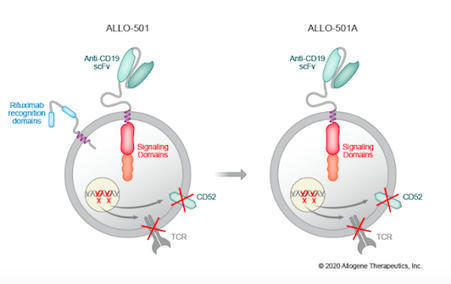
This week’s update looks at a Phase 1/2 study for ALLO-501A, a TALEN-edited CAR T-cell therapy for large B-cell lymphoma.
Study investigates CAR-T cells in mice models with lupus.
The European Biotech News Website
The European Biotech News Website
The European Biotech News Website
|
According to new research in the journal Immunity, T cells have a nuclear receptor doing something very odd-;but very important-;to help them fight pathogens and destroy cancer cells.
A circulating tumor DNA liquid biopsy can predict the likelihood of early disease progression among patients treated with chimeric antigen receptor T-cell therapy for advanced multiple myeloma, study results showed.The noninvasive tool could be used to quantify a patient’s disease burden, according to investigators who presented findings at Tandem Meetings | Transplantation & Cellular
This week’s update looks at P-BCMA-ALLO1, a Cas-CLOVER-edited allogeneic CAR-T candidate that is being developed for the treatment of multiple myeloma.
A new study has found that a novel T cell genetically engineered by University of Arizona Health Sciences researchers is able to target and attack pathogenic T cells that cause Type 1 diabetes, which could lead to new immunotherapy treatments.
Dr Badie said, “I believe these recent results show we have a potential breakthrough treatment that may have a remarkable impact on patients with malignant brain tumors.”
Using CRISPR technology, researchers can modify T cells to attack tumors. It is ready to be tested on 18 patients with advanced stages of myeloma, sarcoma and melanoma at Penn's Perelman School of Medicine; the University of California, San Francisco; and the University of Texas MD Anderson Cancer Center in Houston.
|



 Your new post is loading...
Your new post is loading...





















CAR-T cells have shown clinical efficacy in blood cancers, but have not been as effective in solid tumors. This variation in efficacy is partly due to the fact that tumors promote T-cell depletion, in which the cells are less effective at actively killing cancer. The researchers therefore created a map for the domain of T-cell differentiation and depletion in tumors, based on high-dimensional loss-of-function genetic screening. The researchers thus mapped the transcription factors expressed in tumor-infiltrating T cells. This map will provide a guide that future researchers can refer to and use to identify ways of improving T-cell-based immunotherapies. The researchers achieved this feat using single-cell CRISPR-Cas9 screening, a gene-editing technology that analyzes the gene expression profiling of individual cells after selectively eliminating transcription factors in a comprehensive screen. By examining the gene expression pattern of an individual T lymphocyte, the researchers were able to compare them to determine which inactivated transcription factors most affected T cell differentiation and anti-cancer activity. The same approach could be more widely applicable to increase knowledge in a number of contexts.